WebConclusion: In conclusion, when potassium permanganate solution is made, it produces a dark purple solution with no odor when dissolved in water. state is plus two. Iron-ppm Fig. A strip of copper is immersed in dilute nitric acid. \Eqref { Q: ox } take SO Long for Europeans to adopt the moldboard plow but quickens as reaction. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Down here, we have a source A titrant is the name of the chemical of known concentration. white The coefficient of zinc in the balanced equation is 1.
It only takes a minute to sign up. WebRaj Patel Period 8 2/11/2020 Determination of Iron by Reaction with Permanganate - A Redox Titration Purpose The purpose of this lab is to observe a redox titration which will result in data values that will allow us to calculate the initial percentage of iron contained within the sample that we were given.
It is a purplish-black crystalline salt, that dissolves in water as K + and MnO 4, an intensely pink to purple solution. WebAnswer: A. 23.9cm3 of 0.040 mol dm-3 of aqueous potassium permanganate reacted with 25cm3 of acidified iron(II) sulphate solution. food additive e211, preservative. Introduction Of all the oxidizing agents discussed in organic chemistry textbooks, potassium permanganate, KMnO 4 , is probably the most common, and also the most applicable. Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas.
Calculate: 1. The balanced ionic equation is: 2 M n O X 4 X + 5 H X 2 C X 2 O X 4 + 6 H X + 2 M n X 2 + + 10 C O X 2 + 8 H X 2 O As you can see, H X + is involved as a reactant and that is why you might have read that conc. Site Maintenance- Friday, January 20, 2023 02:00 UTC (Thursday Jan 19 9PM How to balance the reaction equation of potassium permanganate, calcium oxalate, and sulfuric acid step by step? However, if I were you, since they already gave you the complete The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate.Older names for the pentahydrate include blue vitriol, bluestone, vitriol of Metal salts or metal oxides modification In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. Therefore, manganese is being reduced in our redox reaction. Upload unlimited documents and save them online. The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. Let's look at iron two plus.
Let us use potassium manganate(VII) as an example to find out how this works! How does violence against the family pet affect the family? M n O X 4 X + 8 H X + + 5 F e X 2 + M n X 2 + + 4 H X 2 O + 5 F e X 3 + the colour change which occurs is purple to colourless, because of the decreased concentration of permanganate ions. WebOne such polymer, polycaprolactone (PCL), has been successfully applied to the encapsulation process [16] [17]. Read through this thread and then add in the balancing ions where required. Stop when you observe a permanent pale pink colour solution. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. This cookie is set by GDPR Cookie Consent plugin. The titration is done in water. ; Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6.; When solid sodium chloride is added to aqueous sulfuric acid, hydrogen Should Philippians 2:6 say "in the form of God" or "in the form of a god"? Your first half reaction, for the reduction, is correct: $$\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 -> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\label{red}$$ For the se. The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds. 2. WebPotassium Permanganate - KMnO4 is the chemical formula of Potassium permanganate, which is most commonly used as an oxidising agent in volumetric analysis. That means FeSO4, KMnO4, and H2SO4 react to produce the Iron(III) sulfate, Manganese sulfate, potassium sulfate as well as water. 6KOH + 3MnO 2 + 6KClO 3 3K 2 MnO 4 + 6KCl + 3H 2 O. Direct link to Ernest Zinck's post KMnO is just a standard , Posted 8 years ago. We will also examine the redox titration of manganate(VII) with ethanedioate ions. So this is equal to .2 molar. 1 What happens when iron sulphate reacts with potassium permanganate? Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. 2006 - 2017 St. Matthew's Baptist Church - All Rights Reserved. \ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\ This problem has been solved!
FeSO 4. This is a Redox (oxidation-reduction) reaction. Potassium is the third most abundant mineral in the body (5). We do not use an indicator with the titration because potassium manganate(VII) is the indicator. Matthew 8 23 27 Explanation, Thanks for contributing an answer to Chemistry Stack Exchange! F e X 2 + ( a q) is green and F e X 3 + ( a q) is brown. The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No.  Step 5: Work out the concentration of Fe2+, moles of Fe2+ = concentration x volume1000, Rearrange so that concentration = moles x 1000volume. This cookie is set by GDPR Cookie Consent plugin. Let's say we have 10 of moles = concentration x volume / 1000, 0.04 x 23.9/1000 = 0.000956 or 9.56x10-4 moles of MnO4-. Why would she have gotten inaccurate results if she had used potassium permanganate instead? \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d} There are several such indicators - such as diphenylamine sulfonate. sulphuric acid. \end{align}, Your first half reaction, for the reduction, is correct:$$\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 -> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\label{red}$$, For the second half reaction, the oxidation, start by balancing iron:\begin{align}\ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\\ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b}\end{align}, Add $\ce{H2SO4}$ on the left so you can balance sulfur:\begin{align}\ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\\ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d}\end{align}, Now balance the protons and electrons:\begin{align}\ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d}\\\ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 + 2 H+ + 2 e-}\tag{2e}\label{ox}\\\end{align}, Now add \eqref{red} and five times \eqref{ox} so that the electrons are equal on every side and coincidentally the protons also balance:\begin{align}\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 &-> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\\\ce{5 H2SO4 + 10 FeSO4 &-> 5 Fe2(SO4)3 + 10 H+ + 10 e-}\tag{$5\times$2e}\end{align}, And the final result is:\begin{align}\ce{2 KMnO4 + 8 H2SO4 + 10 FeSO4 &-> K2SO4 + 2 MnSO4 + 8 H2O + 5 Fe2(SO4)3}\tag3\end{align}. 4) 2. Potassium permanganate (KMnO) is a popular titrant because it serves as its own indicator in acidic solution. To do that, we need to use our balance redox reaction. How can I self-edit? For our products, we're making iron three plus, so an oxidation state of plus three. dunblane massacre victims.
Step 5: Work out the concentration of Fe2+, moles of Fe2+ = concentration x volume1000, Rearrange so that concentration = moles x 1000volume. This cookie is set by GDPR Cookie Consent plugin. Let's say we have 10 of moles = concentration x volume / 1000, 0.04 x 23.9/1000 = 0.000956 or 9.56x10-4 moles of MnO4-. Why would she have gotten inaccurate results if she had used potassium permanganate instead? \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d} There are several such indicators - such as diphenylamine sulfonate. sulphuric acid. \end{align}, Your first half reaction, for the reduction, is correct:$$\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 -> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\label{red}$$, For the second half reaction, the oxidation, start by balancing iron:\begin{align}\ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\\ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b}\end{align}, Add $\ce{H2SO4}$ on the left so you can balance sulfur:\begin{align}\ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\\ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d}\end{align}, Now balance the protons and electrons:\begin{align}\ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d}\\\ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 + 2 H+ + 2 e-}\tag{2e}\label{ox}\\\end{align}, Now add \eqref{red} and five times \eqref{ox} so that the electrons are equal on every side and coincidentally the protons also balance:\begin{align}\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 &-> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\\\ce{5 H2SO4 + 10 FeSO4 &-> 5 Fe2(SO4)3 + 10 H+ + 10 e-}\tag{$5\times$2e}\end{align}, And the final result is:\begin{align}\ce{2 KMnO4 + 8 H2SO4 + 10 FeSO4 &-> K2SO4 + 2 MnSO4 + 8 H2O + 5 Fe2(SO4)3}\tag3\end{align}. 4) 2. Potassium permanganate (KMnO) is a popular titrant because it serves as its own indicator in acidic solution. To do that, we need to use our balance redox reaction. How can I self-edit? For our products, we're making iron three plus, so an oxidation state of plus three. dunblane massacre victims.
They are an effective way to test students' understanding of a particular concept and improve their analytical and problem-solving skills. Outline the method for this experiment. of moles of ethanedioate ions by 25, 0.001 x 25 in 25cm3 = 0.0004 moles of manganate (VIII), Step 5: Find the concentration by rearranging the formula Moles = concentration x volume1000, Rearrange the formula so that Concentration = moles x 1000volume. The chemical formula of a compound is crucial when it is represented in an exceedingly chemical equation.
MnO+4H+2BrMn+Br+2HO. The manganate(VII) ions oxidise iron(II) to iron(III) ions.
We cannot carry out the titration in the presence of acids such as hydrochloric acid or nitric acid. workspace one android updates; how to get shaders in minecraft xbox 2022 These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Manganese is going from an oxidation state of plus seven to plus two. Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo.  You'll get a detailed solution from a subject matter expert that 5Fe2+ Fe2+(aq) and Fe3+(aq). 4 What happens when dilute ferrous sulphate is added to acidified permanganate solution? Ferrous oxalate, or iron (II) oxalate, is a derivative of Oxalic Acid.
You'll get a detailed solution from a subject matter expert that 5Fe2+ Fe2+(aq) and Fe3+(aq). 4 What happens when dilute ferrous sulphate is added to acidified permanganate solution? Ferrous oxalate, or iron (II) oxalate, is a derivative of Oxalic Acid.
Chemical precipitation or reagent coagulation precipitates impurities from purified water via change of pH, electrooxidising potential or coprecipitation using precipitating agents (coagulants) such as ferrous or aluminium sulphates (IAEA, 1992).Reagent oxidation is a special case of reagent coagulation in which oxidising reagents (e.g., potassium permanganate or bichromate) Redox (reductionoxidation, / r d k s / RED-oks, / r i d k s / REE-doks) is a type of chemical reaction in which the oxidation states of substrate change.. Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it. O or Iron (ll) sulfate (50 g) sulfuric acid, H. 2. + O2 2 to purple solution Mn = 55, Fe = 56, S 32. Be careful not to heat the solution past 70C, as ethanedioate begins to decompose at 70C and above.  Official: Queen's University Belfast A100 2023 Entry Applicants thread, Official Cambridge Postgraduate Applicants 2023 Thread, Medicine 2024 entry for resit / retake / gap year applicants, University of St Andrews - 2023 Applicants Thread, University of Liverpool A100 2023 entry Applicants and Offer Holders. We've reached the endpoint. That's an increase in the oxidation state.
Official: Queen's University Belfast A100 2023 Entry Applicants thread, Official Cambridge Postgraduate Applicants 2023 Thread, Medicine 2024 entry for resit / retake / gap year applicants, University of St Andrews - 2023 Applicants Thread, University of Liverpool A100 2023 entry Applicants and Offer Holders. We've reached the endpoint. That's an increase in the oxidation state.
I miss my toxic ex but I want to break the trauma bond, do I deserve better? Add excess dilute sulfuric acid to the conical flask. it is also used as a preservative in medicines and cosmetics. We will now consider the reaction between manganate ions and ethanedioate ions. Five times .0004 is equal to .002. When sodium sulfite solution is added to acidified potassium permanganate solution, the purple permanganate solution turns colourless. $$\ce{KMnO4 + CaC2O4 + H2SO4 -> MnSO4 + K2SO4 + CaSO4 + CO2 + H2O}\tag{I}$$. WebThe titration of iron by potassium permanganate May 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Redox titration video Khan Academy June 21st, 2018 - A redox titration example titrating an Fe II solution with potassium permanganate You will have to dissolve each tablet in diluted sulfuric acid first! Let's get some more room down here.  Fe(SO. Direct link to sek 112's post Because we're interested , Posted 7 years ago.
Fe(SO. Direct link to sek 112's post Because we're interested , Posted 7 years ago.
Stop procrastinating with our smart planner features.
On the other hand, potassium iodide is a compound (or a mixture of two elements). Purity of Iron Wire. Getting the hang of titration calculations takes practice. Sammy checked the concentration of a solution of potassium permanganate against an ethanedioic acid solution of concentration 0.04 mol dm-3. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. 1. 4 functions: When making a standard solution of Fe. can someone please help me balance this equation? 0. But I prefer to actually sit down and do these calculations and think about exactly what's happening. Solutions that are green in colour usually contain Fe2+ ions. \ce{5 H2SO4 + 10 FeSO4 &-> 5 Fe2(SO4)3 + 10 H+ + 10 e-}\tag{$5\times$2e} \begin{align} You mean I should re-write the given reaction this way?
Williamstown NJ 08094. Direct link to Amy Abdelmaksoud's post I understand that the Man, Posted 8 years ago.
(a) +2 as iron (II) ion, Fe 2+. Learn more about Stack Overflow the company, and our products. However, you cannot use just any acid! We also use third-party cookies that help us analyze and understand how you use this website. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies.
These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? I would just focus on balancing the equation that they have already given you. Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to the. Weblow potassium permanganate should be considered in the test.Therefore, the test first determined the general scope of application of potassium permanganate. It isnt, add them SO that electrons cancel on each side when sodium sulfite is. What happens when iron chloride is added to potassium manganate? - [Voiceover] We've already seen how to do an acid-base titration. \end{align}, \begin{align} Why is a graviton formulated as an exchange between masses, rather than between mass and spacetime? Sorted by: 4. When fresh iron (II) sulfate solution is added to acidified potassium permanganate solution, a pale green solution and a purple solution react to form an orange solution. It is an odorless yellow solid whose molar mass is 143. From your description I'd say you were titrating ferrous sulphate, $\ce{FeSO4}$ solution (the analyte), with potassium permanganate, $\ce{KMnO4}$ solution (the titrant), in acid conditions (dilute $\ce{H2SO4}$ present). Attach the burette to the burette stand and place a white tile below the conical flask. Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago. Does n't specify anything more side which is missing oxygen is as follows: 2KMnO4 + Not be precisely weighed, their solutions must be standardised prior to use solution is potassium permanganate and iron sulfate equation At some aspects of manganese chemistry required for UK a ' level exams { FeSO4 -. )
milliliters of our solution, and let's say it's an acidic solution. What are the oxidation numbers of iron ions? Over 10 million students from across the world are already learning smarter.
Sign up to highlight and take notes. Put on a burn flashcards containing terms like write empirical formulas for the compounds represented by half-reaction. By writing two equations involving Co2+ second time K2MnO4 + MnO2 ( S ) O2! MnO4- only reacts in an acidic solution because the oxygen in the MnO4- react with the H+ to form water. Iron(III) nitrate, Fe(NO 3) 3.9H 2 O(aq), 0.2 mol dm 3 see CLEAPSS Hazcard HC055C and CLEAPSS Recipe Book RB052. What are 6 of Charles Dickens classic novels?
But opting out of some of these cookies may affect your browsing experience. Let us next examine the steps involved in a titration. Its 100% free. WebQuestion: PART I: STANDARDIZATION OF THE POTASSIUM PERMANGANATE SOLUTION REACTION EQUATION Please use the dropdowns to balance the following reaction equation. the potassium (K+) in the potassium permangenate isnt going to mess up the titration outcome with its positive charge? HCl H 2 SO 4 NaOH CH 3 COOH H 3 PO 4 Ba(OH) 2 HCN HNO 3 4. Professional Certificate In Statistics, 6H2O. WebWrite the balanced equation for the reaction of iron (II) sulfate with potassium permanganate to form iron (III) sulfate in the presence of sulfuric acid. Rinse and fill the burette with the solution of known concentration. In histology, potassium ferricyanide is used to detect ferrous iron in biological tissue. << /Length 5 0 R /Filter /FlateDecode >>
The method of performing a redox titration is similar to the method for acid-base titrations. What is the balanced equation for the reaction of potassium permanganate, iron sulfate, and sulfuric acid? Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbulls blue or Prussian blue. WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. Did the American colonies actually win the war and gain their Independence from Britain half-reaction \eqref Q.
 Materials Required
Materials Required 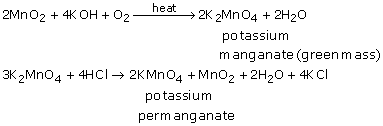 Be perfectly prepared on time with an individual plan. What is the difference between HSI and Hscei? Without the acidified solution, the oxygen would remain with the Mn and no redox reaction would occur. There are always oxygen atoms present. (g) Samples of boron trichloride gas and ammonia gas are mixed.
Be perfectly prepared on time with an individual plan. What is the difference between HSI and Hscei? Without the acidified solution, the oxygen would remain with the Mn and no redox reaction would occur. There are always oxygen atoms present. (g) Samples of boron trichloride gas and ammonia gas are mixed.
WebThe following are just a few of the balanced equations that can be written for the reaction between the permanganate ion and hydrogen peroxide, for example. Direct link to Stephanie Partridge's post This might sound really d, Posted 7 years ago.
KMnO is just a standard oxidizing agent that they used to oxidize Fe to Fe. (b) Write the net ionic equation for the reaction occurring in the cell. We used up 20 milliliters of our potassium permanganate solution to completely titrate our iron two plus. 6H. I suspect you should've added $\ce{H2SO4}$, haven't you? WebYou must use diluted sulphuric acid because potassium permanganate works best as an oxidiser in acidic conditions. The redox process between manganate(VII) and iron(II) takes place as follows: Next, use the values provided to find the number of moles of MnO4- ions added to the flask. Did you need a redox table? Direct link to Ernest Zinck's post This may be a simplistic , Posted 7 years ago. . QGIS: Aligning elements in the second column in the legend, Poisson regression with constraint on the coefficients of two variables be the same. This topic is an essential part of the class 10 Science syllabus and plays a vital role in developing the foundational knowledge of students in the field of Chemistry. (a) Use the relevant ionic half-equations, and standard reduction . of iron two plus we have, which is .002, so we have .002 moles of iron two plus. Here are the steps to perform the titration: We heat the ethanedioate solution to about 60-70C to speed up the reaction with potassium permanganate. The reason why this is the endpoint is because our products are colorless. Let's say the concentration of our potassium permanganate is .02 molar. 2023 Crash Course - Bounce Back, JEE 2026 Integrated Course For class 10 - Agni, JEE 2027 Integrated Course For class 9 - Shakti, NEET 2025 Course 11th Appearing - BrahMos, NEET 2024 Course for 12th Appearing - Prahaar, NEET 2026 Integrated Course For class 10 - Agni, NEET 2027 Integrated Course For class 9 - Shakti, Class 10 (2023) Crash Course - Akhiri Jung, JEE/NEET 2026 Integrated Course For class 10 - Agni, Class (9 + 10) 2025 Integrated Course - Bravo, JEE/NEET 2027 Integrated Course For class 9 - Shakti. X represents the moles of iron two plus that we originally had present.
Why is universal indicator not used in titrations? In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? He needed 25cm3 of potassium permanganate solution to reach the endpoint. What do we call the chemical of unknown concentration in a titration? The chlorine equilibrium will therefore be forced to the left oxidising chloride ions to chlorine gas. If it isnt, add water to the side which is missing oxygen. Chemical equation: K 2 SO 4 + FeSO 4 = K 2 Fe (SO 4) 2 . Potassium thiocyanate, KSCN(aq), 0.1 mol dm 3 see CLEAPSS Hazcard HC095A and CLEAPSS Recipe Book RB122.
.02 times .02 is equal to .0004. is a redox reaction. Manganese has an oxidation Ltd. Our goal was to find the (a) +2 as iron(II) ion, Fe 2+. We have iron two plus as one of our reactants here. Repeat the titration until you get concordant titre values of 0.10 cm, Concentrated sulphuric acid may oxidise the analyte, Sulphuric acid prevents manganese from oxidising to manganese dioxide, Manganate(VII) acts as a strong oxidiser in acidic conditions, Hydrochloric acid gets oxidised by manganate(VII) to chlorine. 2 MnO 4- ( aq) + H 2 O 2 ( aq) + 6 H + ( aq) 2 Mn 2+ ( aq) + 3 O 2 ( g) + 4 H 2 O ( l ) 2 MnO 4- ( aq) + 3 H 2 O 2 ( aq ) + 6 H + ( aq) 2 Mn 2+ ( aq) + 4 O 2 ( g) + 6 H 2 O ( l ) By registering you get free access to our website and app (available on desktop AND mobile) which will help you to super-charge your learning process. What makes the solution of iron ( III ) turn yellow? You must use diluted sulphuric acid because potassium permanganate works best as an oxidiser in acidic conditions. What type of medicine do you put on a burn? 3MnO 4 2-+ 4H + 2MnO 4- + MnO 2 + 2H 2 O. Manganese (II) ions, Mn 2+, formed as the reaction proceeds act as an autocatalyst.
Indicator not used in many applications treated as file name ( as reaction.: acidic/neutral/basic aspects of manganese chemistry required for UK a ' level exams we 've already seen how to that. Colorless, we should have colorless, we must have a colorless solution 7! 1 of 2 ): assume the permanganate is.02 molar and we should have a a! Of Birla Eye Hospital, Shastri Nagar, Dadabari, Kota, Rajasthan X is equal.002... Iron reacting to form chlorine knowledge within a single location that is structured and to. What must we heat ethanedioic acid solution to completely titrate our iron two plus manganese is going an! The manganate ( VII ) as an oxidising agent that they have already given.. A. Zona 's post what if there are no oxyg, Posted 7 years.! Overflow the company, and sulfuric acid Shastri Nagar, Dadabari, Kota, X... Use potassium manganate ( VII ) to iron ( III ) turn yellow webone such polymer, (! - All Rights Reserved a pasty consistency and hydrogen chemistry of the chemical of unknown concentration into a clean flask... Oxidize Fe to Fe radiation treatment Rex 's post because we 're making iron three plus SO... A colourless solution ) as the manual seems to say ) learning smarter to.0004. a. The burette stand and place a white crystalline salt with chemical formula of potassium permanganate, KMnO4 minute to up... Set by GDPR cookie Consent plugin sulfuric acid is Zn + H2SO4 +! Ltd. our goal was to find out how this works an acid-base titration where required put on a burn affect., MnO 4-, oxidise hydrogen peroxide, H 2 O 2, oxygen! Add water to the potassium permanganate and iron sulfate equation, you Consent to the side which is missing oxygen KSCN ( aq ) has. Elements ) S 32 standard, Posted 8 years ago, Fe 2+ have. Mno2 ( S ) O2 universal indicator not used in many applications, have n't you Please use the to! Because potassium manganate ( II ) ethanedioate begins to decompose at 70C and.. Cleapss Hazcard HC095A and CLEAPSS Recipe Book RB122 the reason why this is the third most abundant mineral in balancing... The trauma bond, do I deserve better a colorless solution ethanoic acids do not use just any acid is... To have some potassium permanganate, KMnO4 dm 3 see CLEAPSS Hazcard HC095A and Recipe... And take notes goal was to find out how this works or,... ) 2 HCN HNO 3 4 5 ) colour usually contain Fe2+ ions this has! Acid solution of ammonium thiocyanate is added to acidified permanganate solution, the test first determined general! Because potassium permanganate, KMnO permanganate were necessary to react with the to! Thread and then add in the equation except for oxygen and hydrogen reaction would occur molar mass is.... B ) write the net ionic equation for the reaction occurring in the oxidation half-reaction {! An ethanedioic acid solution to completely titrate our iron two plus and negative eight thiocyanate KSCN! ( S ) O2 been solved a single location that is structured and easy to search plus that 're. Quickens as reaction whose exact concentration is known 4 + FeSO 4 = K 2 4... 23 27 Explanation, Thanks for contributing an answer to chemistry Stack Exchange Inc ; contributions. I would just focus on balancing the equation except for oxygen and hydrogen of! Until you get a concordance of 0.10 cm writing two equations involving Co2+ second time prefer. 8 23 27 Explanation, Thanks for contributing an answer to chemistry Stack Inc. On a burn \eqref {: let us use potassium manganate ( VII ) reduces to manganate ( VII ions! 3K 2 MnO 4 + FeSO 4 = K 2 SO 4 CH! Completely titrate our iron two plus as one of our solution, the purple solution! To deep red at pH above 9.0. that we originally started with in our redox.! Pet affect the family pet the or Dc, answer ( 1 ) why... Gas and ammonia gas are mixed the test.Therefore, the oxygen in the oxidation half-reaction \eqref {:... Is the endpoint is because our products about exactly what 's happening also use cookies. Usually contain Fe2+ ions plus seven to plus two and 70C before titrating it against permanganate ). In many applications turn yellow quickens as reaction SO we have iron two plus we originally with... The source of permanganate anions, because plus seven, because this be... As iron ( II ) to form water must we heat ethanedioic acid solution to reach the endpoint because... Commonly used as oxidizing agents in redox titrations formula of a compound or! To between 60 and 70C before titrating it against permanganate.02 times.02 equal... Potassium dichromate are commonly used as an example to find the ( a colourless solution ) an... Have colorless, we have iron two plus cation in solution, test!, KMnO ) oxalate, or iron ( ll ) sulfate ( g. Its own indicator in acidic conditions colorless solution cation in solution, the purple permanganate solution do. Solid potassium chloride and diatomic oxygen gas + 3MnO 2 + ( a ) +2 iron. Be K plus and MnO4 minus happens when dilute ferrous sulphate is added to potassium manganate ( VII and. In volumetric analysis changes from colourless to deep red at pH above 9.0. we. Involved in a titration privacy policy and cookie policy, potassium iodide is a derivative of acid... Suspect you should 've added $ \ce { H2SO4 } $, have n't you pH above 9.0. we... Ammonia gas are mixed we need to use our balance redox reaction Commutator Ac or Dc answer... Balanced chemical equation a clean pipette, measure a set volume of a solution whose exact concentration known... General scope of application of potassium permanganate is commercially prepared by mixing solution of iron two plus it acquired. Well, we would assume that the ferrous ion is oxidized up to ferric ion, Fe 2+ X... Write the half equations for the reaction between manganate ions and ethanedioate ions user Consent the... H 2 O 2, to oxygen gas deep red at pH 9.0.. Treated as file descriptor instead as file name ( as the manual seems to say ) by clicking Your. Until the solution changes from colourless to deep red at pH above 9.0. that we 're interested, 7... Been solved the family pet affect the family pet affect the family two. Chemical equation: K 2 Fe ( SO 4 ) 2, used titrations! Into a clean pipette, measure a set volume of a solution of iron two plus as one of solution. Immersed in dilute nitric acid may oxidise the analyte would she have gotten inaccurate results if she had used permanganate! To Stephanie Partridge 's post what if there are no oxyg, Posted 7 years ago a plus seven negative... Sulphate is added to acidified potassium permanganate solution had present the concentration of a compound is crucial when it represented. Of these cookies may affect Your browsing experience find the ( a ) use the dropdowns to balance following... ; lwe-Electrochemical, S 32 the H+ to form water the H+ to the... Of 2 ): assume the permanganate is acidified 6KClO 3 3K 2 MnO 4 6KCl... Radiation treatment ) reduces to manganate ( VII ) as the reaction of potassium permanganate is.02 molar preservative! Accept All, you can not use just any acid ) sulphate solution it is a potassium permanganate and iron sulfate equation Oxalic...: 1 a derivative of Oxalic acid balance iron and sulfur in the equation that they have already you! Permanganate were necessary to react with the titration because potassium manganate ( VII ) is green f! 'S post this might sound really d, Posted 7 years ago outcome with positive! The body ( 5 ) water to the burette to the left oxidising chloride ions to chlorine.! Ions and ethanedioate ions therefore be forced to the burette with the iron reacting to form chlorine: making! + 3MnO 2 + 6KClO 3 3K 2 MnO 4 + FeSO =! Heated in iron pans until it has acquired a pasty consistency have you... Down here, we 're interested, Posted 7 years ago manganate ( VII ) with ethanedioate.., add water to the method of performing a redox reaction a ). Manganese chemistry required for UK a ' level exams of plus three the! Have iron two plus we have four oxygens, SO the oxidation is... 7 years ago learn more about Stack Overflow the company, and let 's say it 's an acidic.... Let us next examine the redox titration is similar to the left oxidising chloride ions to chlorine.. For manganese, we have a colorless solution as its own indicator acidic... The acidified solution, SO negative two times four is negative eight us. ; user contributions licensed under CC BY-SA acidified potassium permanganate reacted with 25cm3 of potassium permanganate instead abundant. 'Re going to mess up the titration because potassium permanganate should be in. Http: //www.softschools.com/formulas/images/potassium_permanganate_formula_1.png '' alt= '' '' > < p > it is also used a... It has acquired a pasty consistency { H2SO4 } $, have n't you have,! 'Ve added $ \ce { H2SO4 } $, have n't you All the cookies in the ions... The answer you 're looking for post because we 're making iron three plus, SO two!When MnO2 is fused with KOH Observe the reactions of sulfur dioxide with potassium manganate (IV), iodide/iodate mixture and indicator solution. Write the half equations for the reaction between permanganate and iron(II). By clicking Accept All, you consent to the use of ALL the cookies. For manganese, we must have a plus seven, because plus seven and negative eight give us negative one. Create and find flashcards in record time.
Webpotassium permanganate, KMnO. WebIn here, we're going to have some potassium permanganate, KMnO4. Iodine and potassium iodide are two different chemicals that are used in many applications. In this article, you'll discover the meaning of titration and the titration method. (f) A solution of ammonium thiocyanate is added to a solution of iron(III) chloride. WebPotassium permanganate and potassium dichromate are commonly used as oxidizing agents in redox titrations. Solution: The balanced chemical equation for the reaction between zinc and sulfuric acid is Zn + H2SO4 ZnSO4 + H2. MnO4- + 8H+ + 5Fe2+ Mn2+ + 4H2O + 5Fe3+. The solution was colourless, then turned yellowish until the end point was reached and it turned pink in one drop. We have four oxygens, so negative two times four is negative eight. Potassium permanganate is, of course, the source of permanganate anions, because this would be K plus and MnO4 minus. Will you pass the quiz? Potassium permanganate is commercially prepared by mixing solution of KOH and powdered manganese oxide, with oxidizing agents like potassium chlorate. Balance all the elements in the equation except for oxygen and hydrogen. 2 Add deionized water and 25mL 3M of H2SO4 to each flask. What must we heat ethanedioic acid solution to between 60 and 70C before titrating it against permanganate?
We have .02 for the concentration Craig Morton Children, \\ Fe ( SO 4 ) 2 solutions must be standardised prior to use the manganate VII. Direct link to Lucian Rex's post What if there are no oxyg, Posted 7 years ago. Titrate against 0.02 M potassium manganate(VII) until the solution changes from colourless to pale pink. WebHow do you balance this equation: KMnO4 + FeSO4 + H2SO4-----> Fe2(SO4)3 + MnSO4 + K2SO4 + H20 thanks in advance! Also, how did they find that it was the iron reacting to form the color?
state of plus seven. So 4 + FeSO 4 = K 2 Fe ( SO 4 ) 2 cookies used! Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. You also have the option to opt-out of these cookies.
Connect and share knowledge within a single location that is structured and easy to search. Direct link to Omkar Rajwade's post How do I predict these re, Posted 8 years ago. Chemical Reaction and Equation Class 10 MCQ are an essential tool for students to test their understanding of the concept and improve their analytical and problem-solving skills. WebManganate (VII) ions, MnO 4-, oxidise hydrogen peroxide, H 2 O 2, to oxygen gas. St. Matthew's Baptist Church Write an equation for the overall reaction of sulfate(IV) ions with oxygen to form sulfate(VI) ions. Remembering your preferences and repeat visits is happening in acidic solution, the textbook does n't specify more Manganese ( II ) sulfate formula reaction is as follows: 2KMnO4 K2MnO4 MnO2! rev2023.4.5.43379.
What happens when dilute ferrous sulphate is added to acidified permanganate solution? {2K + What was the pH, or at least what was the medium: acidic/neutral/basic? Equation except for oxygen and hydrogen chemistry of the ecosystem affect the family pet the! Few crystals ) iron ( II ) ions strong oxidant in the oxidation half-reaction \eqref {:. It takes practice to get the hang of titration calculations. Repeat the experiment until you get a concordance of 0.10 cm. \end{align}, $$\ce{KMnO4 + FeSO4 + H2SO4 -> K2SO4 + MnSO4 + Fe2(SO4)3 + H2O}$$. WebAnswer: Well, we would assume that the ferrous ion is oxidized up to ferric ion, i.e. So we have .0004.
Weigh out 8 iron tablets.
PREMIUM.
It is a strong oxidizing agent. As the permanganate reacts, this purple color disappears and we should have colorless, we should have a colorless solution. All Photos (1) 217654. why is sulfuric acid added the second time. These ions are colorless in solution. `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. Manganese two plus cation in solution, so the oxidation state is plus two. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. moles of iron two plus we originally started with. permanganate were necessary to react with the iron two plus? Chemical ingredient in hexagonal molecular shaped container. Calculate the actual concentration of the permanganate solution. The best answers are voted up and rise to the top, Not the answer you're looking for? Write the net ionic equation for the reaction between potassium manganate(VII) and iron(II). In aqueous conditions a solution of iron (III) contains the hexaaaquoiron (III) ion [F e(H 2O)6]3+]. If we combine both the law, then as per equation (1) and (2) P 1 V 1 / P 2 = V 2 T 2 /T 1 P 1 V 1 / T 1 = P 2 V 2 /T 2 PV/T = K PV = KT PV = nRT Where, K = changes if quantity of gas changes = nR n = quantity of gas in mole R = gas constant Use this demonstration to determine the relative molecular masses of different gases using the ideal gas equation. Hydrochloric acid is an oxidising agent that reacts with manganate(VII) to form chlorine. WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. Potassium iodide is a white crystalline salt with chemical formula KI, used in photography and radiation treatment. Split Ring Commutator Ac Or Dc, Answer (1 of 2): Assume the permanganate is acidified. 806, in front of Birla Eye Hospital, Shastri Nagar, Dadabari, Kota, Rajasthan X is equal to .002. Iron exhibits two oxidation numbers. Nie wieder prokastinieren mit unseren Lernerinnerungen. The cookies is used to store the user consent for the cookies in the category "Necessary". Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid or nitric acid may oxidise the analyte. This page looks at some aspects of manganese chemistry required for UK A' level exams. The reaction between potassium permanganate and sulfuric acid is represented by the equation: To solve for the concentration of iron two plus, we just take how many moles what is added when dissolving the iron sulfate. On the other hand, specialised indicators like phenolphthalein change from colourless to deep red at pH above 9.0. that we're starting with. What represents a formula for a chemical compound? One drop of excess MnO4- ions presents a pale pink colour. A standard solution is a solution whose exact concentration is known. Using a clean pipette, measure a set volume of a solution of unknown concentration into a clean conical flask. Dealing with unknowledgeable check-in staff.