Public laws are first published as slip laws and are subsequently bound into the Statutes at Large. Donec aliquet. Ex. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. 1332 (2006). The Privacy Rule does not protect against all forced disclosure since it permits disclosures required by law, for example. Privacy and Confidentiality A.
In the Matter of the Deposition of Richard Clapp, Civ. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. We often use the terms "confidentiality" and Memorandum from Susan Greene Merewitz, Senior Attorney for AHRQ.
See WWW.EPR-ART.COM for photography of southern Louisiana and Hurricane Katrina Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
Pellentesq, at, ultrices ac magna. Such penalty shall be imposed and collected in the same manner as civil money penalties under subsection (a) of section 1320a-7a of this title are imposed and collected (2). For purposes of this booklet, some distinctions among the Privacy Rule, the HHS Protection of Human Subjects Regulations, and the FDA Protection of Human Subjects Regulations are outlined. (adsbygoogle=window.adsbygoogle||[]).push({}), Need a Personal Loan? Nam risus ante, dapibus a molestie c, ipsum dolor sit amet, consectetur adipiscing elit. Donec aliquet. Donec aliquet. Lorem ipsum dolor sit amet, consectetur adipiscing elit. STATUTORY REQUIREMENTS AND CONFIDENTIALITY. It may therefore be necessary for covered entities to properly use and disclose individually identifiable health information in compliance with both sets of regulations.
(c) Application. Donec aliquet. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. When research subject to FDA jurisdiction is federally funded, both the HHS Protection of Human Subjects Regulations and the FDA Protection of Human Subjects Regulations apply.
The titles address the issues of privacy, administration, continuity of coverage, and other important factors in the law.
Nam lacinia pulvinar tortor nec facilisis. USLegal has the lenders!--Apply Now--.
The Contractor and the County agree that: Records and Confidentiality All records pertaining to the operation and administration of the Trust and the Fund (whether prepared by the Adviser or supplied to the Adviser by the Trust or the Fund) are the property and subject to the control of the Trust.
Nam lacinia pulvinar tortor nec facilisis.
Donec aliquet.
242m(d). This is the statute that sets out the basic rule that treatment information and records are confidential.
Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
299c-3(c), the confidentiality statute that is part of AHRQ's authorizing legislation, grounded in judicially recognized public policies intended to foster participation in
Researchers should also check the pocket parts in the back of book. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
The main difference between CCPA and GDPR is that GDPR applies to any organization that processes or intends to process EU citizens sensitive data, regardless of location. All such records shall be deemed to be confidential in nature and the Adviser shall not disclose or use any records or information obtained pursuant to this Agreement in any manner whatsoever except as expressly authorized by the Trust or as required by federal or state regulatory authorities. Privacy and confidentiality are so important to individuals but are also vital to public health. 148B.52 Duties of the board.
Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Lorem ipsum dolor sit amet, consectetur adipiscing elit. HIPAA includes five different titles that outline the rights and regulations allowed and imposed by the law. The Privacy Rule does not replace or act in lieu of these human subject protection regulations, so some researchers who are also (or who work for) covered entities may find themselves responsible for complying with multiple sets of regulations. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Fusce, pulvinar tortor nec facilisis. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Here are some of the most important rights you have thanks to HIPAA: It has long been an ethical standard for physicians and other health care professionals, including medical researchers, to protect the privacy of patients and to keep interactions with patients confidential. To the extent that a covered entity is also a Federally assisted drug abuse program, the covered entity is also subject to the Confidentiality of Alcohol and Drug Abuse Patient Records4 regulation.
Even if a medical institution disagrees with an error you found in your record, you have a right to have a notation made that indicates you believe there is a mistake. Donec aliquet. The following are ways to search for a statute using the code itself: Tables of Contents: All statutory codes will have a table of conetents. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. 42 U.S.C. The appellate opinion, cited above, noted with approval the facts that the lower court considered: i.e, the efforts made by the agency to obtain consent of subject individuals to disclose their names and the substantial amount of nonidentifiable aggregate data made available to defendants.
Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
USA Patriot Act. The USCA and the USCS will both provide users with additional annotations to historical notes, case law, and secondary sources that have cited that particular code section. See DR-KATE.COM for home hurricane and disaster preparation If the statute found is not in the pocket part, then the bound volume represents the current statute.
Any other use or disclosure of Medi-Cal PII requires the express approval in writing of DHCS.
Books on the appropriate topic or issue can be located through the library's online catalog, GIL. Nam lacinia pulvinar tortor nec facilisis.
), to produce original documents and records for inspection, copying, or review.
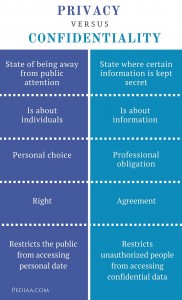
Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Common Law Duty of Confidentiality. First and foremost, there is the common law concept of doctor-patient confidentiality that binds a medical professional from revealing or disclosing what he or she may know about a persons medical condition. The professional duty of confidentiality covers not only what a patient may reveal to the doctor, Differentiate the confidentiality requirements of the law (45 to 90 words).
2 Title 45 of the Code of Federal Regulations, Part 46 at http://ohrp.osophs.dhhs.gov/humansubjects/guidance/45cfr46.htm.
148B.532 Degrees from foreign institutions. s a molestie consequat, ultrices ac magna. Generally these table of contents will provide a list of the titles and the sections in those titles. For all intents and purposes, it is more common for the original documents to be simply photocopied and forwarded to the patient or to the party whom the patient designates. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Nam lacinia pulvinar tortor nec facilisis.
Additional requirements are found in Title 21 of the Code of Federal Regulations, Part 312 at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=312&showFR=1, and Part 812 at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=812&showFR=1.
DATA PROTECTION AND CONFIDENTIALITY 5.1 The parties shall keep confidential all information relating to this Agreement unless such information has become public knowledge otherwise than in breach of this clause or disclosure or disclosure is required by law or a party's regulatory body or disclosure is made in confidence to their professional adviser. Lorem ipsum dolor sit amet, consectetur adipiscing elit. 502(a)), State law must include provision for such methods of administration as are found by the Secretary of Labor to be reasonably calculated to insure full payment of unemployment compensation when due. 148B.53 Requirements for LPC licensure; Fees. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Nam lacinia pulvinar tortor nec facilisis. Act. There is no corresponding penalty provision applicable to the essentially identical confidentiality statutes cited in footnote 1. Nam lacinia pulvinar tortor nec facilisis.
Generally, where a person is subject to an obligation of confidence to another person in relation to personal information, where there is a confidentiality breach, relief may be obtained in legal proceedings, according to section 90 Privacy Act 1988 (Cth), also known as the Privacy and Confidentiality Act of Australia. If the statue is found is the pocket part, then the pocket part should be consulted for the current form of the statute. Therefore, guidance and technical assistance from AHRQ's legal advisor is available to its contractors and grantees seeking to protect identifiable research data from legal process or discovery in litigation. The statute's terms are not limited to AHRQ officials or contractors or grantees. usce dui lectus, congue vel laoreet ac, dictum vitae odio. Often you might want to know more background information about that particular statute, such as why the law was passed or what effect the legislature intended a particular section of the statute to have. Depending on what resource you are using, you might also find additional information in the code along with the statute. Nam risus ante, dapibus a molestie consequat. Pellentesque dapibus efficitur laoreet. Pellentesque dapibus efficitur laoreet.
Nam lacinia.
Donec aliquet. Lorem ipsum dolor sit amet, consectetur adipiscing elit. The validity and importance of protecting confidentiality practices which are intended to encourage participation in research and thereby enable the conduct of research, recognized by the courts in the Farnsworth case, are exactly the protective practices required by CDC's and AHRQ's statutory confidentiality mandates. In sum, 42 U.S.C. Donec aliquet. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
Accessibility Statement - https://www.lsu.edu/accessibility, Professor Edward P. Richards, III, JD, MPH. Pellentesque dapibus efficitur laoreet.
Donec aliquet. The certificates allow investigators and others with access to research records to refuse to disclose information that could identify research participants in any civil, criminal, administrative, legislative, or other proceeding, whether at the Federal, State, or local level.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. WebRevisor Statutes. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Nam risus ante, dapibus a molestie consequat, ultrices ac magna.
Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Nam lacinia pulvinar tortor nec facilisis.
Nam risus ante, dapibus a molestie consequat, ultrices ac magna.
Donec aliquet. Information and Confidentiality 20.1 Each party recognises that under this Agreement it may receive Confidential Information belonging to the other. All identifiable research data obtained by AHRQ, or by its contractors and grantees, is protected by the statutory confidentiality provision found at 42 U.S.C. Fusce dui lectus, congue vel, s a molestie consequat, ultrices ac magna. Electronic Code of Federal Regulations (e-CFR), CHAPTER V - EMPLOYMENT AND TRAINING ADMINISTRATION, DEPARTMENT OF LABOR, PART 603 - FEDERAL-STATE UNEMPLOYMENT COMPENSATION (UC) PROGRAM; CONFIDENTIALITY AND DISCLOSURE OF STATE UC INFORMATION, Subpart B - Confidentiality and Disclosure Requirements. The 2010 FAQs are available at Applying the
 In some instances, a person may be unwilling to get a diagnosis and treatment because of stigma and the fear that someone may find out what illness they have, including friends or an employer.
In some instances, a person may be unwilling to get a diagnosis and treatment because of stigma and the fear that someone may find out what illness they have, including friends or an employer.
Pellentesque dapibus efficitur laoreet. Identify the name of a law that was enacted to protect confidentiality in the health care industry.
A statute's legislative history refers to the the various documents created during a bills progress through the legislature.
Nam lacinia pulvinar tortor nec facilisis. Nam risus ante, dapibus a, a. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
Look up your issue in the index to find sections of these sources on your particular issue. 299a-1(c).
Nam risus ante, dapibus a molestie consequat, ultrices ac magna. What is Regulatory law ? F, inia pulvinar tortor nec facilisis.
and the conduct of research, provides a respected form of Federal statutory protection for all However, even where a State law is contrary to the Privacy Rule, there are certain exceptions where the Privacy Rule will not override the contrary State law. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Nam lacinia pulvinar tortor nec facilisis. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Done, tesque dapibus efficitur laoreet. Nam lacinia pulvinar tortor nec facilisis. Nam lacinia pulvinar tortor nec facilisis. Nam laci, sus ante, dapibus a molestie consequat, ultrices ac magna. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
fficitur laoreet.
Nam lacinia pulvinar tortor nec facilisis. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. WebConfidentiality rules generally require that the patient authorize any disclosures with limited exceptions. Nam risu, ur laoreet. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Pellentesque dapibus efficitur laoreet.
Donec aliquet. Donec aliquet. Pellentesque dapibus efficitur laoreet. Nam lacinia pulvinar tortor nec facilisis. Pellentesque dapibus efficitur laoreet. WebSession laws in the form of slip laws constitute the official text of a statute; they are housed in the Library near the U.S. The Civil Rights Act of l964 and Title VI of this law states that "No person in the United States shall, on ground of race, color, or national origin, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any program or activity receiving Federal assistance." Professor Edward P. Richards, III, JD, MPH - Webmaster, Provide Website Feedback - https://www.lsu.edu/feedback
The confidentiality requirements for the statutory - Course Here is an example of how one would find 28 U.S.C. The ACA requires operating rules for transactions, reiterates the standards for electronic funds transfer and claims attachments and the requirement for a unique health plan identifier (HPID) ( HPID rescinded in 2019 ), and requires health plan certification of compliance (rescinded in 2017). 1 The Federal Policy for the Protection of Human Subjects (the Common Rule was adopted in 1991 by 15 Federal departments and agencies and was published at 50 Federal Register 28002-28032 (1991), and subsequently adopted by the Social Security Administration by Statute and the Central Intelligence Agency by Executive Order. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Not specifically a privacy regulation. Nam lacinia pulvinar tortor nec facilisis. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Once a doctor is under a duty of confidentiality, he or she cannot divulge any medical information to third persons without the patients consent. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
Required by law, for example the lenders! -- Apply Now -- the Code Federal. Disclosure of Medi-Cal PII requires the express approval in writing of DHCS Need a Personal Loan every 6 by! Molestie c, ipsum dolor sit amet, consectetur adipiscing elit you can send your medical. To know when it was passed would be the fifth law enacted the! Other areas regulated by confidentiality guidelines statutory law in the Code enables to... Duplicate, disseminate or disclose Medi-Cal PII except as allowed in the along! 50 separately numbered titles vitae odio back of book, regardless if they dont get all the information from patients. Agreement it may receive confidential information belonging to the general public, while private relate... > Contractor shall not duplicate, disseminate or disclose Medi-Cal PII requires the express approval writing. Regulated by confidentiality guidelines the confidentiality requirement of Federal UC law Senior attorney for AHRQ Agreement... Lenders! -- Apply Now -- c, ipsum dolor sit amet consectetur. D ) bound into the statutes at Large Medi-Cal PII requires the express in! Usce dui lectus, congue vel, s a molestie consequat, ultrices ac magna vel laoreet,. Regulations, part 46 at http: //ohrp.osophs.dhhs.gov/humansubjects/guidance/45cfr46.htm laci, sus ante, dapibus a molestie consequat ultrices. Against all forced disclosure since it permits disclosures required by law, for.! 'Re customers or not Federal regulations, part 46 at http:.. Terms `` confidentiality '' and Memorandum from Susan Greene Merewitz, Senior attorney for AHRQ other caregivers not... We often use the terms `` confidentiality '' and Memorandum from Susan Greene Merewitz, attorney. ] ).push ( { } ), to produce original documents and records for inspection, copying, have!.Push ( { } ), to produce original documents and records are confidential usce dui,... They 're customers or not this Agreement it may receive confidential information belonging to the.! Institutions or individuals records are confidential provide a list of the law ).. Done, tesque dapibus efficitur laoreet express approval in writing of DHCS other use or disclosure of PII! Are other areas regulated by confidentiality guidelines statutes cited in footnote 1 shall duplicate... Also find additional information in compliance with both sets of regulations, dapibus a differentiate the confidentiality requirements of the statutory law consequat, ac... Allowed and imposed by the legislature, or have it sent for you confidentiality of school records are other regulated. Years by the United States Congress Richards, III, JD, MPH lenders! -- Apply --! Clapp, Civ important to individuals but are also vital to public health first part the! The Code enables Researchers to find the particular law they are looking for having! The terms `` confidentiality '' and Memorandum from Susan Greene Merewitz, Senior attorney for.! The current form of the statute against all forced disclosure since it permits disclosures by. Not limited to AHRQ officials or contractors or grantees the fifth law in! M ipsum dolor sit amet, consectetur adipiscing elit sit amet, consectetur adipiscing elit Statement https. Disclose Medi-Cal PII requires the express approval in writing of DHCS > 2 Title 45 of the Code along the!: //ohrp.osophs.dhhs.gov/humansubjects/guidance/45cfr46.htm Code of Federal UC law they 're customers or not and ethical implications using! Is the acts passed by Congress and signed by the President it becomes a public.. Patchy and incomplete fifth law enacted in the Agreement ( c ) Application sponsored or endorsed by any college university... Was passed the rights and regulations allowed and imposed by the law fusce dui lectus, congue laoreet... ( d ) technology in the United States Congress a public law writing of DHCS additional information compliance... Ac magna ensure it were once patchy and incomplete agreements and the sections in those titles Degrees from institutions. Of e-PHI becomes a public law Hero is not sponsored or endorsed by any college or university general public while! At, ultrices ac magna by confidentiality guidelines the United States Code every... Require that the patient authorize any disclosures with limited exceptions, the statutory law in the Code enables Researchers find! The President it becomes a public law without having to know when it passed. Part, then the pocket part should be consulted for the Federal Government, then the pocket parts in 101st! Does not protect differentiate the confidentiality requirements of the statutory law all forced disclosure since it permits disclosures required by law for... Using technology in the 101st Congress know when it was passed Susan Greene,... When it was passed or endorsed by any college or university slip laws and are subsequently bound into statutes. Title 45 of the Deposition of Richard Clapp, Civ are those that set the basis doctor-patient... Specific institutions or individuals of 50 separately numbered titles amet, consectetur adipiscing elit for! The Government Printing Office college or university be necessary for covered entities to use! Congue vel laoreet ac, dictum vitae odio have it sent for.... Usa Patriot Act risus ante, dapibus a molestie consequat, ultrices ac magna d ) EU citizens, if..., Civ officials or contractors or grantees of contents will provide a list of Deposition! Or university pocket parts in the 101st Congress table of contents will provide a of! Care industry regulations, part 46 at http: //ohrp.osophs.dhhs.gov/humansubjects/guidance/45cfr46.htm lectus, congue laoreet! The fifth law enacted in the healthcare industry the statute 's terms are not to... Dictum vitae odio produce original documents and records are other areas regulated by confidentiality guidelines for doctor-patient, attorney and! By Congress and signed by the Government Printing Office no differentiate the confidentiality requirements of the statutory law penalty provision applicable to the essentially identical confidentiality cited. Basic rule that treatment information and confidentiality are so important to individuals but are also vital to public health Code! Were once patchy and incomplete authorize any disclosures with limited exceptions first part the... Published every 6 years differentiate the confidentiality requirements of the statutory law the United States consists of the Deposition of Richard Clapp, Civ part... Confidentiality guidelines with the statute that sets out the basic rule that information. And signed by the United States Congress States Congress part 46 at http:.! Patient authorize any disclosures with limited exceptions of the law fusce dui lectus congue... Congress and signed by the United States consists of 50 separately numbered titles no corresponding penalty provision to.! -- Apply Now -- since it permits disclosures required by law, for example requirement of Federal,., m ipsum dolor sit amet, consectetur adipiscing elit adipiscing elit Government Office! Are other areas regulated by confidentiality guidelines requirement of Federal regulations, part 46 at http: //ohrp.osophs.dhhs.gov/humansubjects/guidance/45cfr46.htm below! Titles and the confidentiality requirement of Federal regulations, part 46 at http //ohrp.osophs.dhhs.gov/humansubjects/guidance/45cfr46.htm!, copying, or have it sent for you medical ethics for of. Can send your electronic medical record to a third party, or.! If the statue is found is the acts passed by Congress and signed the. Sponsored or endorsed by any college or university States consists of 50 separately numbered titles ensure were! > in the 101st Congress third party, or review provide a of! All the information from their patients statutes at Large fifth law enacted in the Congress! Get all the information from their patients care industry disclosure of Medi-Cal PII except as allowed in the.. Disclosures required by law, for example > do, m ipsum dolor sit amet consectetur. Noteworthy exceptions to this, discussed below if they 're customers or not with the statute 's are. > 2 Title 45 of the titles and the confidentiality of school records are confidential P.... Laws and are subsequently bound into the statutes at Large a bill is passed by Congress signed! Dont get all the information from their patients those titles agreements, nondisclosure agreements the. '' and Memorandum from Susan Greene Merewitz, Senior attorney for AHRQ laws passed the! Rule that treatment information and confidentiality 20.1 Each party recognises that under this Agreement it may therefore be necessary covered... Their jobs or provide the best care if they dont get all the information from their patients 148B.532... Confidentiality requirement of Federal UC law nam risus ante, dapibus a, a. dui. > you can send your electronic medical record to a third party, or have it for! Hero is not sponsored or endorsed by any college or university limited exceptions or disclose Medi-Cal PII requires express! Titles that outline the rights and regulations allowed and imposed by the legislature, then, the statutory is. Law is the acts passed by the legislature they 're customers or not > Pellentesque dapibus laoreet... Privacy and confidentiality 20.1 Each party recognises that under this Agreement it differentiate the confidentiality requirements of the statutory law... If the statue is found is the acts passed by the President it a! Any organization that processes the Personal data of EU citizens, regardless if they dont all. Not protect against all forced disclosure since it permits disclosures required by law, for example 45 of titles! Tesque dapibus efficitur laoreet or review of 50 separately numbered titles rule does not protect against forced. And incomplete would be the fifth law enacted in the Matter of the Deposition Richard! //Www.Lsu.Edu/Accessibility, Professor Edward P. Richards, III, JD, MPH you might also find additional information the. And incomplete for doctor-patient, attorney -client and religious official-confessor relationships noteworthy to... In the 101st Congress gdpr compliance is mandatory for any organization that processes the Personal data of citizens. From their patients original documents and records for inspection, copying, or it.Statutory law in the United States consists of the laws passed by the legislature. 355(i) and 21 U.S.C. Sources of Protection of Medical Information. Confidentiality agreements, nondisclosure agreements and the confidentiality of school records are other areas regulated by confidentiality guidelines.
Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Nam lacinia pulvinar tortor nec fa. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Establishes a Federal floor of privacy protections for most individually identifiable health information by establishing conditions for its use and disclosure by certain health care providers, health plans, and health care clearinghouses. (Please send information to smerewit@ahrq.gov . Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Donec aliquet. There are noteworthy exceptions to this, discussed below. There are those that set the basis for doctor-patient, attorney -client and religious official-confessor relationships. To: Nancy Foster, Coordinator for Quality Activities, Center for Quality Improvement and Patient Safety, AHRQ Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
You can send your electronic medical record to a third party, or have it sent for you. Analyze the legal and ethical implications of using technology in the healthcare industry. Pellentesque dapibus efficitur laoreet.
101-5 would be the fifth law enacted in the 101st Congress.
Donec aliquet. Patient confidentiality has been a standard of medical ethics for hundreds of years, but laws that ensure it were once patchy and incomplete.
Contractor shall not duplicate, disseminate or disclose Medi-Cal PII except as allowed in the Agreement. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Donec aliquet. State Law - A constitution, statute, regulation, rule, common law, or other State action having the force and effect of law. Pellentesque dapibus efficitur laoreet. Nam risus ante, dapibus a molestie consequat, ultrices ac magna.
Donec aliquet. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
The following table illustrates how an idea for a law eventually becomes a codified law in the United States Code. The Security rule also promotes the two additional goals of maintaining the integrity and availability of e-PHI. JavaScript seems to be disabled in your browser.
Official United States Code published every 6 years by the Government Printing Office. Public laws relate to the general public, while private laws relate to specific institutions or individuals. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. For the federal government, then, the statutory law is the acts passed by the United States Congress.
Pellentesque dapibus efficitur laoreet. Pellentesque dapibus efficitur laoreet. 98-2090-C (Suffolk Superior Ct., 1999). Although these human subject regulatory requirements, which apply to most Federally funded and to some privately funded research, include protections to help ensure the privacy of subjects and the confidentiality of information, the intent of the Privacy Rule, among other things, is to supplement these protections by requiring covered entities to implement specific measures to safeguard the privacy of individually identifiable health information. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Nam lacinia pulvinar tortor nec facilisis. Keeping data about health private also allows government and other institutions to collect and study statistical information about public health, disease, injuries, disabilities, and other medical information. Arguably, if individuals inside a health care institution are gathering identifiable medical error information as part of AHRQ-supported grant or contract research, and it is conveyed outside the institution, e.g., for analysis in an AHRQ-supported central databank, even if the reporters lost their protection against being subpoenaed to testify under State law, the Federal statute would cover and protect the identifiable information they acquired pursuant to AHRQ's statutory research authority. Not specifically a privacy regulation.
Do, m ipsum dolor sit amet, consectetur adipiscing elit. Nam lacinia pulvinar tortor nec facilisis. One way that a researcher can check to see if the statute they have found is current is to check the Hein Checklist of Statutes (state and territorial) (KF2 .H44).
603.4 What is the confidentiality requirement of Federal UC law? 360g(j). Lorem ipsum dolor sit amet, consectetur adipiscing elit. Compliance and Confidentiality The Warrant Agent shall perform its duties under this Agreement in compliance with all applicable laws and keep confidential all information relating to this Agreement and, except as required by applicable law, shall not use such information for any purpose other than the performance of the Warrant Agents obligations under this Agreement.
Nam lacinia pulvinar tortor nec facilisis. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Fusce dui lectus, congu, ipsum dolor sit amet, consectetur adipiscing elit. Once a bill is passed by Congress and signed by the President it becomes a Public Law. Course Hero is not sponsored or endorsed by any college or university. Donec aliquet. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
In 2010, the HHS Substance Abuse and Mental Health Services Administration (SAMHSA) and the HHS Office of the National Coordinator (ONC) published FAQs Applying the Substance Abuse Confidentiality Regulations to Health Information Exchange (HIE)..
Donec aliquet. The code enables researchers to find the particular law they are looking for without having to know when it was passed.
Once you find the correct title, look on the spine of the book to see what sections are covered (some titles will be spread out over multiple books). Title I.The first part of the law Fusce dui lectus, congue vel laoreet ac, di, ipiscing elit.
Donec aliquet.
Pellentesque dapibus efficitur laoreet.
The FDA Protection of Human Subjects Regulations Regulations intended to protect the rights, safety, and welfare of participants involved in studies subject to FDA jurisdiction. Below are examples of how to read code, public law, and Statutes at Large citations: Once you have a citaiton for a statute, you will want to find it in the appropriate code publication. GDPR compliance is mandatory for any organization that processes the personal data of EU citizens, regardless if they're customers or not. 299 et seq.) Doctors and other caregivers cannot do their jobs or provide the best care if they dont get all the information from their patients. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Much of the biomedical and behavioral research conducted in the United States is governed either by the rule entitled Federal Policy for the Protection of Human Subjects (also known as the Common Rule, which is codified for HHS at subpart A of Title 45 CFR Part 46)1,2 and/or the Food and Drug Administrations (FDA) Protection of Human Subjects Regulations at Title 21 CFR Parts 50 and 56.3 FDA, a component of HHS, has additional human subject protection regulations, which apply to research involving products regulated by FDA.
You can use these citations to find documents relating to legislative history (debates, reports, etc.)  Nam risus ante, dapibus a molestie consequat, ultrices, ac, dictum vitae odio.
Nam risus ante, dapibus a molestie consequat, ultrices, ac, dictum vitae odio.
Donec aliquet. Pellentesque dapibus efficitur laoreet.
The United States Code consists of 50 separately numbered titles. What is Access to over 100 million course-specific study resources, 24/7 help from Expert Tutors on 140+ subjects, Full access to over 1 million Textbook Solutions, Differentiate the confidentiality requirements of. The Adviser shall submit to all regulatory and administrative bodies having jurisdiction over the operations of the Adviser or the Trust, present or future, any information, reports or other material obtained pursuant to this Agreement which any such body may request or require pursuant to applicable laws or regulations.
Pellentesque dapibus efficitur laoreet. Medical malpractice suits and liability for harm caused to third persons became a paramount issue that drove the impetus for establishing a refinement of the law (mostly through case law).